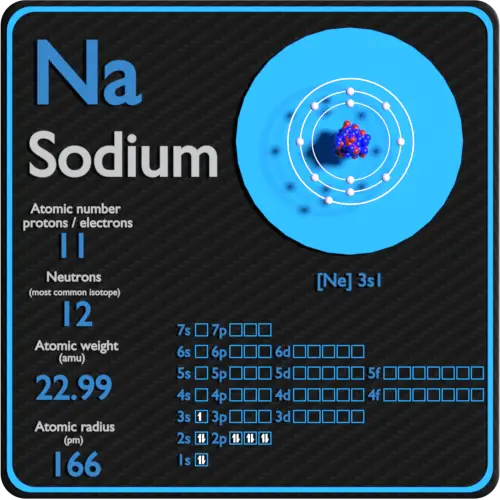
Na Electrons
Most atoms do not have eight electrons in their valence electron shell. Some atoms have only a few electrons in their outer shell, while some atoms lack only one or two electrons to have an octet. In cases where an atom has three or fewer valence electrons, the atom may lose those valence electrons quite easily until what remains is a lower shell that contains an octet. Atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the positive charges of the protons in the nucleus. Positively charged ions are called cations. Most metals become cations when they make ionic compounds.
Na Element Electrons
1.) Determine the number of 3s electrons in Na. Express your answer as an integer. 2.) Determine the number of 3d electrons in V. Express your answer as an integer. 3.) Determine the number of 4d electrons in Y. Express your answer as an integer. 4.) Determine the number of 6p electrons in Pb. Express your answer as an integer. Valence electrons are those electrons that reside in the outermost shell surrounding an atomic nucleus.
Cations
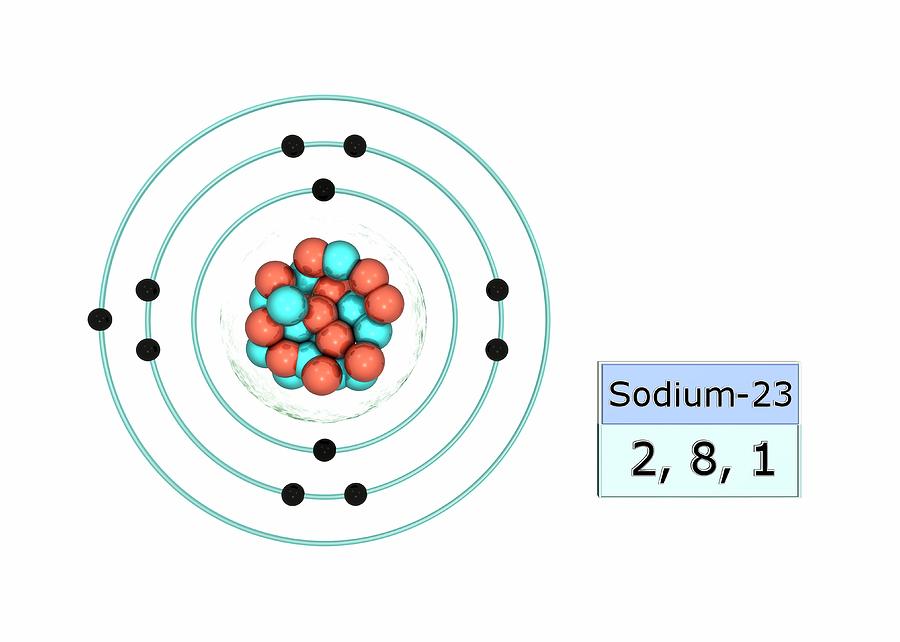
A neutral sodium atom is likely to achieve an octet in its outermost shell by losing its one valence electron.
[ce{Na rightarrow Na^{+} + e^{-}}]
The cation produced in this way, Na+, is called the sodium ion to distinguish it from the element. The outermost shell of the sodium ion is the second electron shell, which has eight electrons in it. The octet rule has been satisfied. Figure (PageIndex{1}) is a graphical depiction of this process.
Anions
Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. Negatively charged ions are called anions. Most nonmetals become anions when they make ionic compounds.
A neutral chlorine atom has seven electrons in its outermost shell. Only one more electron is needed to achieve an octet in chlorine’s valence shell. (In table salt, this electron comes from the sodium atom.)
[ce{e^{-} +Cl -> Cl^{-}}]

In this case, the ion has the same outermost shell as the original atom, but now that shell has eight electrons in it. Once again, the octet rule has been satisfied. The resulting anion, Cl−, is called the chloride ion; note the slight change in the suffix (-ide instead of -ine) to create the name of this anion. Figure (PageIndex{2}) is a graphical depiction of this process.
Na Electrons And Protons
The names for positive and negative ions are pronounced CAT-eye-ons and ANN-eye-ons, respectively.
In many cases, elements that belong to the same group (vertical column) on the periodic table form ions with the same charge because they have the same number of valence electrons. Thus, the periodic table becomes a tool for remembering the charges on many ions. For example, all ions made from alkali metals, the first column on the periodic table, have a 1+ charge. Ions made from alkaline earth metals, the second group on the periodic table, have a 2+ charge. On the other side of the periodic table, the next-to-last column, the halogens, form ions having a 1− charge. Figure (PageIndex{3}) shows how the charge on many ions can be predicted by the location of an element on the periodic table. Note the convention of first writing the number and then the sign on a ion with multiple charges. The barium cation is written Ba2+, not Ba+2.
Contributions & Attributions
This page was constructed from content via the following contributor(s) and edited (topically or extensively) by the LibreTexts development team to meet platform style, presentation, and quality:
Na Electron Configuration
Marisa Alviar-Agnew (Sacramento City College)
Henry Agnew (UC Davis)
