Sulfur Atom
Sulfur is mentioned 15 times in the Bible, and was best known for destroying Sodom and Gomorrah.It was also known to the ancient Greeks, and burnt as a fumigant. Sulfur was mined near Mount Etna in Sicily and used for bleaching cloth and preserving wine, both of which involved burning it to form sulfur dioxide, and allowing this to be absorbed by wet clothes or the grape juice. Name: Sulfur Symbol: S Atomic Number: 16 Atomic Mass: 32.066 amu Melting Point: 112.8 °C (385.95 K, 235.04001 °F) Boiling Point: 444.6 °C (717.75 K, 832.28 °F) Number of Protons/Electrons: 16 Number of Neutrons: 16 Classification: Non-metal Crystal Structure: Orthorhombic Density @ 293 K: 2.07 g/cm 3 Color: yellow British Spelling: Sulphur.
Study Notes
The chemistry of sulfur‑containing organic compounds is often omitted from introductory organic chemistry courses. However, we have included a short section on these compounds, not for the sake of increasing the amount of material to be digested, but because much of the chemistry of these substances can be predicted from a knowledge of their oxygen‑containing analogues.
A thiol is a compound which contains an $ce{-}$SH functional group. The $ce{-}$SH group itself is called a mercapto group. A disulfide is a compound containing an $ce{-}$S$ce{-}$S$ce{-}$ linkage.
(Organic) sulfides have the structure R$ce{-}$S$ce{-}$R′, and are therefore the sulfur analogues of ethers. The nomenclature of sulfides can be easily understood if one understands the nomenclature of the corresponding ethers. Notice that the term “thio” is also used in inorganic chemistry. For example, SO42− is the sulfate ion; while S2O32−, in which one of the oxygen atoms of a sulfate ion has been replaced by a sulfur atom, is called thiosulfate.
Thiolate anions, RS$ce{-}$, are analogous to alkoxy anions, RO$ce{-}$. Thiolate anions are better nucleophiles than are alkoxy anions.
If you have trouble understanding why trialkylsulfonium ions are formed, think of them as being somewhat similar to the hydronium ions that are formed by protonating water:
Later we shall see examples of tetraalkylammonium ions, R4N+, which again may be regarded as being similar to hydronium ions.
Sulfoxides and sulfones are obtained by oxidizing organic sulfides. You need not memorize the methods used to carry out these oxidations.
Table 18.1, below, provides a quick comparison of oxygen‑containing and sulfur‑containing organic compounds.
| Oxygen‑containing Compound | Sulfur Analogue |
|---|---|
| ether, R$ce{-}$O$ce{-}$R′ | sulfide, R$ce{-}$S$ce{-}$R′ |
| R$ce{-}$O$ce{-}$, alkoxy group | R$ce{-}$S$ce{-}$, alkylthio group |
| R$ce{-}$O−, alkoxy anion | R$ce{-}$S−, thiolate anion |
| alcohol, R$ce{-}$OH | thiol, R$ce{-}$SH |
| $ce{-}$OH, hydroxy group | $ce{-}$SH, mercapto group |
| R$ce{-}$O$ce{-}$O$ce{-}$R′, peroxide | R$ce{-}$S$ce{-}$S$ce{-}$R′, disulfide |
Note that when we name thiols, we include the “e” of the alkane name. Thus, CH3CH2SH is called “ethanethiol,” not “ethanthiol.”
The Element Sulfur
Sulfur
| General | |
|---|---|
| Name, Symbol, Number | sulfur, S, 16 |
| Chemical series | nonmetals |
| Group, Period, Block | 16 (VIA), 3 , p |
| Density, Hardness | 1960 kg/m3, 2 |
| Appearance | lemon yellow |
| Atomic properties | |
| Atomic weight | 32.065 amu |
| Atomic radius (calc.) | 100 pm (88 pm) |
| Covalent radius | 102 pm |
| van der Waals radius | 180 pm |
| Electron configuration | [Ne]3s2 3p4 |
| e- 's per energy level | 2, 8, 6 |
| Oxidation states (Oxide) | ±2,4,6 (strong acid) |
| Crystal structure | orthorhombic |
| Physical properties | |
| State of matter | solid |
| Melting point | 388.36 K (239.38 °F) |
| Boiling point | 717.87 K (832.5 °F) |
| Molar volume | 15.53 ×10-6 m3/mol |
| Heat of vaporization | no data |
| Heat of fusion | 1.7175 kJ/mol |
| Vapor pressure | 2.65 E-20 Pa at 388 K |
| Speed of sound | __ m/s at 293.15 K |
| Miscellaneous | |
| Electronegativity | 2.58 (Pauling scale) |
| Specific heat capacity | 710 J/(kg*K) |
| Electrical conductivity | 5.0 E-22 106/m ohm |
| Thermal conductivity | 0.269 W/(m*K) |
| 1st ionization potential | 999.6 kJ/mol |
| 2nd ionization potential | 2252 kJ/mol |
| 3rd ionization potential | 3357 kJ/mol |
| 4th ionization potential | 4556 kJ/mol |
| 5th ionization potential | 7004.3 kJ/mol |
| 6th ionization potential | 8495.8 kJ/mol |
| SI units & STP are used except where noted. | |
Sulfur (sulphur) is a chemical element in the periodic table that has the symbol S and atomic number 16. An abundant tasteless odorless multivalent non-metal, sulfur is best known as yellow crystals and occurs in many sulfide and sulfate minerals and even in its native form (especially in volcanic regions). It is an essential element in all living organisms and is needed in several amino acids and hence in many proteins. It is primarily used in fertilizers but is also widely used in gunpowder, laxatives, matches and insecticides.
Notable characteristics
 This non-metal is pale yellow in appearance, soft, light, with a distinct odor when allied with hydrogen (rotten egg smell). It burns with a blue flame that emits a peculiar suffocating odor (sulfur dioxide, SO2). Sulfur is insoluble in water but soluble in carbon disulfide. Common oxidation states of sulfur include -2, +2, +4 and +6. In all states, solid, liquid, and gaseous, sulfur has allotropic forms, whose relationships are not completely understood. Crystalline sulfur can be shown to form an 8 membered sulfur ring, S8.
This non-metal is pale yellow in appearance, soft, light, with a distinct odor when allied with hydrogen (rotten egg smell). It burns with a blue flame that emits a peculiar suffocating odor (sulfur dioxide, SO2). Sulfur is insoluble in water but soluble in carbon disulfide. Common oxidation states of sulfur include -2, +2, +4 and +6. In all states, solid, liquid, and gaseous, sulfur has allotropic forms, whose relationships are not completely understood. Crystalline sulfur can be shown to form an 8 membered sulfur ring, S8. Sulfur can be obtained in two crystalline modifications, in orthorhombic octahedra, or in monoclinic prisms, the former of which is the more stable at ordinary temperatures.
Applications
It is used for many industrial processes such as the production of sulfuric acid (H2SO4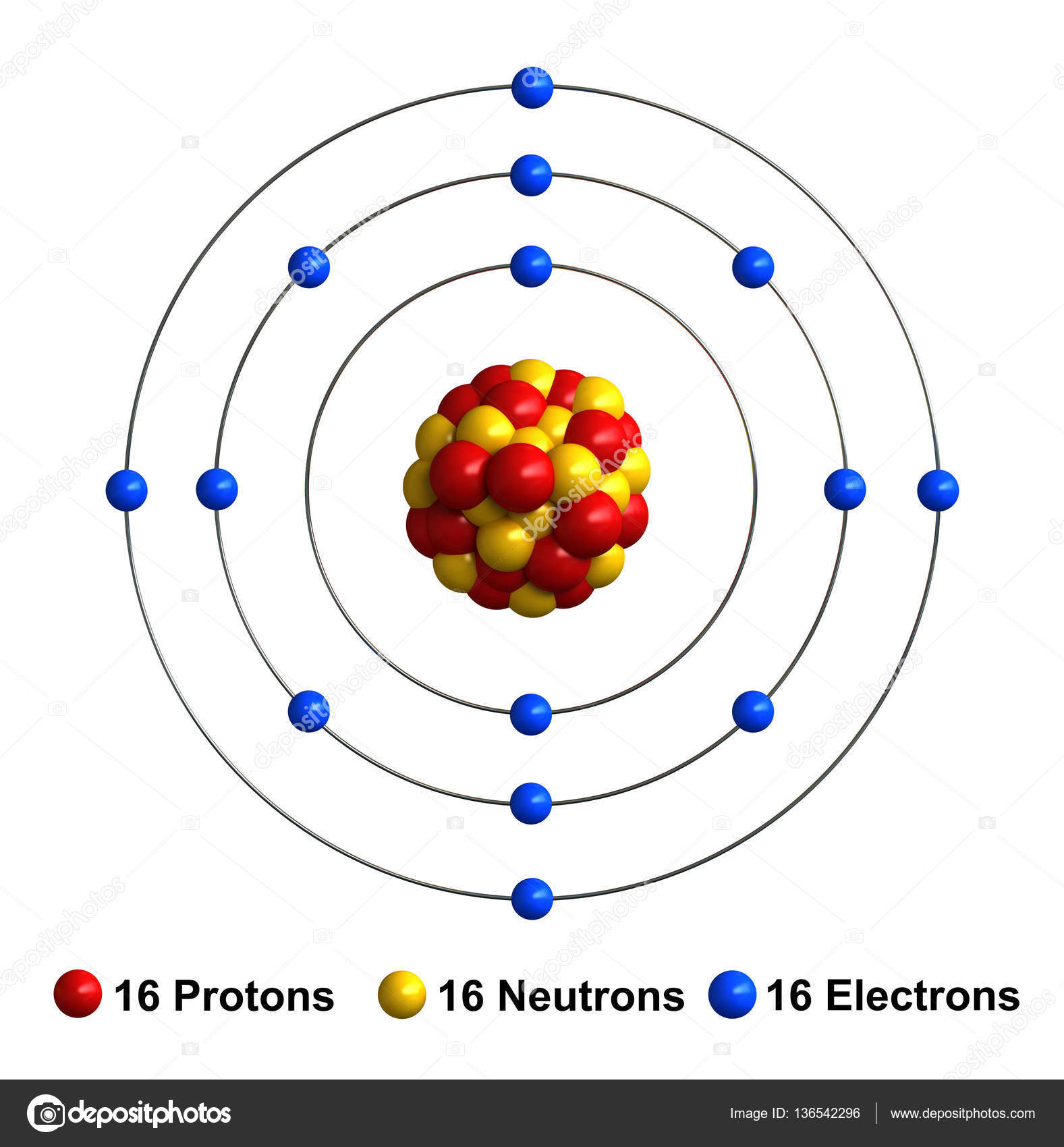
 ) for batteries, the production of gunpowder, and the vulcanization of rubber. Sulfur is used as a fungicide, and in the manufacture of phosphate fertilizers. Sulfites are used to bleach papers and dried fruits. Sulfur also finds use in matches and fireworks. Sodium or ammonium thiosulfate are used as photographic fixing agents. Epsom salts, magnesium sulfate, can be used as a laxative, as a bath additive, as an exfoliant, or a magnesium supplement in plant nutrition.
) for batteries, the production of gunpowder, and the vulcanization of rubber. Sulfur is used as a fungicide, and in the manufacture of phosphate fertilizers. Sulfites are used to bleach papers and dried fruits. Sulfur also finds use in matches and fireworks. Sodium or ammonium thiosulfate are used as photographic fixing agents. Epsom salts, magnesium sulfate, can be used as a laxative, as a bath additive, as an exfoliant, or a magnesium supplement in plant nutrition. Biological role
The amino acids cysteine, methionine, homocysteine, and taurine contain sulfur, as do some common enzymes, making sulfur a necessary component of all living cells. Disulfide bonds between polypeptides are very important in protein assembly and structure. Some forms of bacteria use hydrogen sulfide (H2S) in the place of water as the electron doner in a primitive photosynthesis-like process. Sulfur is absorbed by plants from soil as sulfate ion. Inorganic sulfur forms a part of iron-sulfur clusters, and sulfur is the bridging ligand in the CuA site of cytochrome c oxidase.History
Sulfur (Sanskrit, sulvere; Latin sulpur) was known in ancient times and was called brimstone in the Biblical story of Pentateuch (Genesis). Homer mentioned 'pest-averting sulfur' in the 9th century BC and in 424 BC, the tribe of Bootier destroyed the walls of a city by burning a mixture of coal, sulfur, and tar under them. Sometime in the 12th century, the Chinese invented gun powder which is a mixture of potassium nitrate (KNO3), carbon, and sulfur. Early alchemists gave sulfur its own alchemical symbol which was a triangle at the top of a cross. Through experimentation, alchemists knew that the element mercury can be combined with sulfur. In the late 1770s, Antoine Lavoisier helped convince the scientific community that sulfur was an element and not a compound.
Occurrence
Sulfur occurs naturally in large quantities compounded to other elements in sulfides (example: pyrites) and sulfates (example: gypsum). It is found in its free form near hot springs and volcanic regions and in ores like cinnabar, galena, sphalerite and stibnite. This element is also found in small amounts in coal and petroleum, which produce sulfur dioxide when burned. Fuel standards increasingly require sulfur to be extracted from fossil fuels because sulfur dioxide combines with water droplets to produce acid rain. This extracted sulfur is then refined and represents a large portion of sulfur production. It is also mined along the US Gulf coast, by pumping hot water into sulfur containing deposits (such as salt domes) which melts the sulfur. The molten sulfur is then pumped to the surface. Through its major derivative, sulfuric acid, sulfur ranks as one of the more-important elements used as an industrial raw material. It is of prime importance to every sector of the world's industrial and fertilizer complexes. Sulfuric acid production is the major end use for sulfur, and consumption of sulfuric acid has been regarded as one of the best indexes of a nation's industrial development. More sulfuric acid is produced in the United States every year than any other chemical.The distinctive colors of Jupiter's volcanic moon Io, are from various forms of multen, solid and gaseous sulfur. There is also a dark area near the Lunar crater Aristarchus that may be a sulfur deposit. Sulfur is also present in many types of meteorites.
Compounds
 Many of the unpleasant odors of organic matter are based on sulfur-containing compounds such as hydrogen sulfide, which has the characteristic smell of rotten eggs. Dissolved in water, hydrogen sulfide is acidic (pKa1 = 7.00, pKa2 = 12.92) and will react with metals to form a series of metal sulfides. Natural metal sulfides are found, especially those of iron. Iron sulfides are called iron pyrites, the so called fool's gold
Many of the unpleasant odors of organic matter are based on sulfur-containing compounds such as hydrogen sulfide, which has the characteristic smell of rotten eggs. Dissolved in water, hydrogen sulfide is acidic (pKa1 = 7.00, pKa2 = 12.92) and will react with metals to form a series of metal sulfides. Natural metal sulfides are found, especially those of iron. Iron sulfides are called iron pyrites, the so called fool's goldSulfur Atom Size
. Interestingly, pyrites can show semiconductor properties.[1] Galena, a naturally occurring lead sulfide (as the detector in a 'cat's hair' rectifier) was of course the original semiconductor discovered.Polymeric sulfur nitride has metallic properties even though it doesn't contain any metal atoms. This compound also has unusual electrical and optical properties. Amorphous or 'plastic' sulfur is produced through fast cooling crystalline sulfur. X-ray studies show that the amorphous form may have an eight atom per spiral helical structure
Other important compounds of sulfur include:
- sodium dithionite, Na2S2O4, a powerful reducing agent.
- sulfurous acid, H2SO3, created by dissolving SO2 in water. Sulfurous acid and the corresponding sulfites are fairly strong reducing agents. Other compounds derived from SO2 include the pyrosulfite ion (S2O52-).
- The thiosulfates (S2O32-). Thiosulfates are used in photographic fixing, are oxidizing agents, and ammonium thiosulfate is being investigated as a cyanide replacement in leaching gold.[2]
- Compounds of dithionic acid (H2S2O6)
- The polythionic acids, (H2SnO6), where n can range from 3 to 80.
- The sulfates, the salts of sulfuric acid. Epsom salts are magnesium sulfate.
- sulfuric acid reacting with SO3 in equimolar ratios forms pyrosulfuric acid.
- peroxymonosulfuric acid and peroxydisulfuric acids, made from the action of SO3 on concentrated H2O2, and H2SO4 on concentrated H2O2 respectively.
- thiocyanogen, (SCN)2.
- tetrasulfur tetranitride S4N4.
- A thiol is a molecule with an -SH functional group. These are the sulfur equivalents of alcohols.
- A thiolate ion has an -S- functional group attached. These are the sulfur equivalent of alkoxide ions.
- A sulfide is a molecule with the form R-S-R', where R and R' are organic groups. These are the sulfur equivalents of ethers.
Isotopes
Sulfur
Sulfur has 18 isotopes, of which four stable isotopes: S-32 (95.02%), S-33 (0.75%), S-34 (4.21%), and S-36 (0.02%). Other than 35 S, the radioactive isotopes of sulfur are all short lived. Sulfur-35 is formed from cosmic ray spallation of argon- 40 in the atmosphere. it has a half-life of 87 days.
S, the radioactive isotopes of sulfur are all short lived. Sulfur-35 is formed from cosmic ray spallation of argon- 40 in the atmosphere. it has a half-life of 87 days. When sulfide minerals are precipitated, isotopic equilibration among solids and liquid may cause small differences in the dS-34 values of co-genetic minerals. The differences between minerals can be used to estimate the temperature of equilibration. The dC-13 and dS-34 of co-existing carbonates and sulfides can be used to determine the pH and oxygen fugacity of the ore-bearing fluid during ore formation.
In most forest ecosystems, sulfate is derived mostly from the atmosphere; weathering of ore minerals and evaporites also contributes some sulfur. Sulfur with a distinctive isotopic composition has been used to identify pollution sources, and enriched sulfur has been added as a tracer in hydologic studies. Differences in the natural abundances can also be used in systems where there is sufficient variation in the S-34 of ecosystem components. Rocky Mountain lakes thought to be dominated by atmospheric sources of sulfate have been found to have different dS-34 values from lakes believed to be dominated by watershed sources of sulfate.
Precautions
Carbon disulfide, hydrogen sulfide, and sulfur dioxide should all be handled with care. In addition to being quite toxic (more toxic than cyanide), sulfur dioxide reacts with atmospheric water to produce acid rain. In high atmospheric concentration, it reacts with water in the lungs to form sulfuric acid there; this causes immediate bleeding, the lungs fill up with blood and suffocation results. In creatures without lungs such as insects or plants, it otherwise prevents respiration. Although very smelly in low concentrations, in higher concentrations sulfur quickly deadens the sense of smell, so potential victims may be unaware of its presence until they experience its possibly deadly effects.Spelling
The element is traditionally spelled sulfur in the US and Canada, but sulphur in Britain, New Zealand, and Australia. The IUPAC has adopted the spelling 'sulfur', as has the Royal Society of Chemistry Nomenclature Committee.